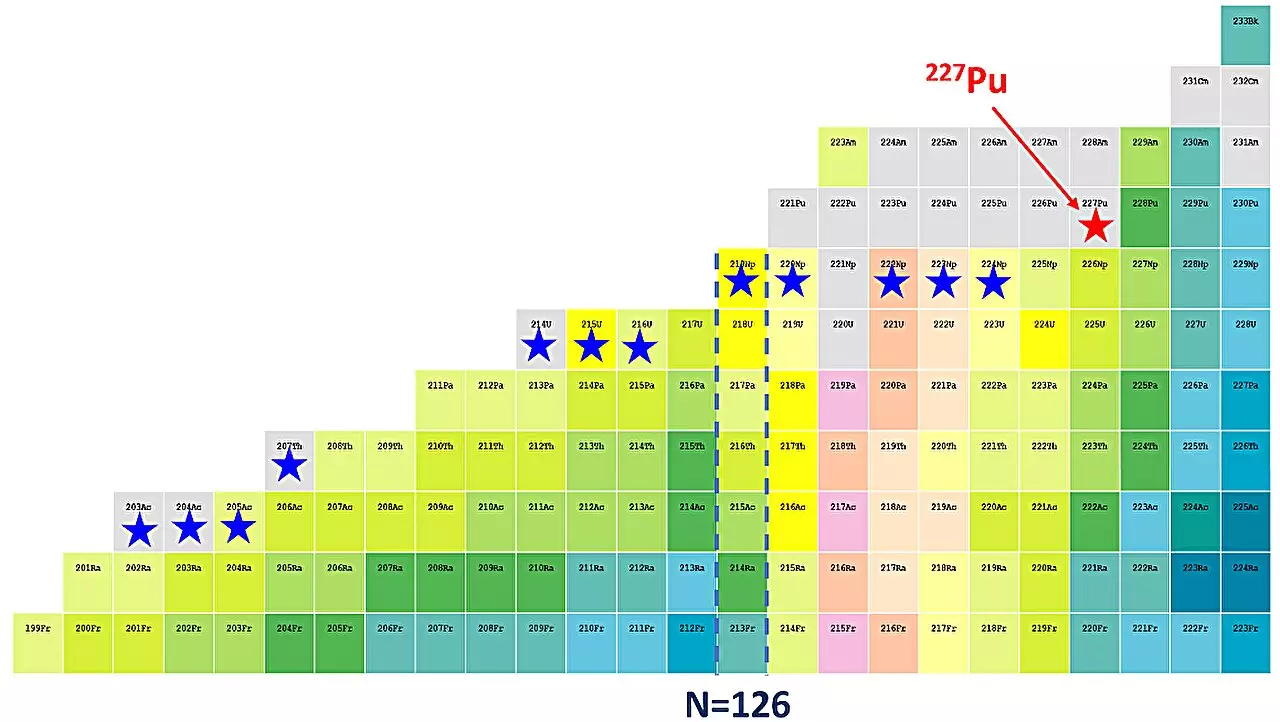
Recent advancements in nuclear physics have opened new frontiers in our understanding of atomic structure. A research team at the Institute of Modern Physics (IMP), part of the Chinese Academy of Sciences (CAS), achieved a substantial breakthrough by synthesizing plutonium-227. As documented in the journal *Physical Review C*, this discovery not only marks the first plutonium isotope identified by Chinese scientists but also contributes to a deeper comprehension of nuclear shell structures.
In nuclear physics, “magic numbers” refer to specific quantities of protons and neutrons that result in notably stable atomic nuclei. The recognized magic numbers—such as 2, 8, 20, 28, 50, 82, and 126—indicate shell closures that confer additional stability. Past research has indicated a weakening of these shell closures, particularly for the neutron shell closure at 126, extending until uranium. This has led scientists to question whether similar phenomena are at play in the region of transuranium elements, where plutonium resides.
To investigate these intriguing questions, the team employed sophisticated experimental techniques at the Heavy Ion Research Facility in Lanzhou, China. Utilizing a gas-filled recoil separator known as the Spectrometer for Heavy Atoms and Nuclear Structure, the researchers executed a fusion evaporation reaction to successfully create plutonium-227. The findings revealed that this isotope is notably neutron-deficient, which has significant implications for our understanding of uranium and other transuranium isotopes.
Plutonium-227 is the 39th isotope uncovered by the IMP team. Through rigorous assessment of nine decay chains, the researchers determined the energy of its alpha particles to be approximately 8191 keV and estimated its half-life at 0.78 seconds. These characteristics were found to align closely with existing data on known plutonium isotopes, suggesting that the shell structure may still hold under these new conditions.
The study’s lead researcher, Prof. Gan Zaiguo, expressed the necessity of further explorations into the robustness of shell closures in plutonium. As indicated by the researchers, plutonium-227 lies seven neutrons away from the magic number of 126. A comprehensive examination of even lighter isotopes—specifically plutonium-221 through plutonium-226—will be crucial for elucidating the dynamics of shell evolution.
Dr. Yang Huabin, the first author involved in the research, emphasized the importance of these findings for advancing our theoretical understanding of nuclear structure. “The synthesis of plutonium-227 is a promising step towards unraveling the complexities of nuclear stability and the role of magic numbers,” he noted.
The discovery of plutonium-227 by the IMP research team is a pivotal moment in nuclear physics, highlighting the continuous evolution of atomic theory. As researchers delve deeper into the world of isotopes, particularly those beyond uranium, they edge closer to answering fundamental questions about nuclear stability and shell closures. This critical investigation not only enhances the body of scientific knowledge but also paves the way for future innovations in nuclear science and technology.

Leave a Reply