Fuel cells have long been considered a promising energy-conversion solution that can generate electricity through electrochemical reactions without contributing to air pollution. These cells have the potential to power a wide range of technologies, from electric vehicles to industrial machinery. However, a significant barrier to their widespread adoption has been the reliance on expensive materials and precious metal catalysts in many fuel cell designs.
While fuel cells offer numerous advantages, the high cost of materials and catalysts has hindered their mass deployment. Anion-exchange-membrane fuel cells (AEMFCs) present a potential solution to these challenges, as they are based on Earth-abundant, low-cost catalysts. Despite the promise of AEMFCs, most non-precious metals used as catalysts in existing devices are susceptible to self-oxidation, leading to irreversible cell failure.
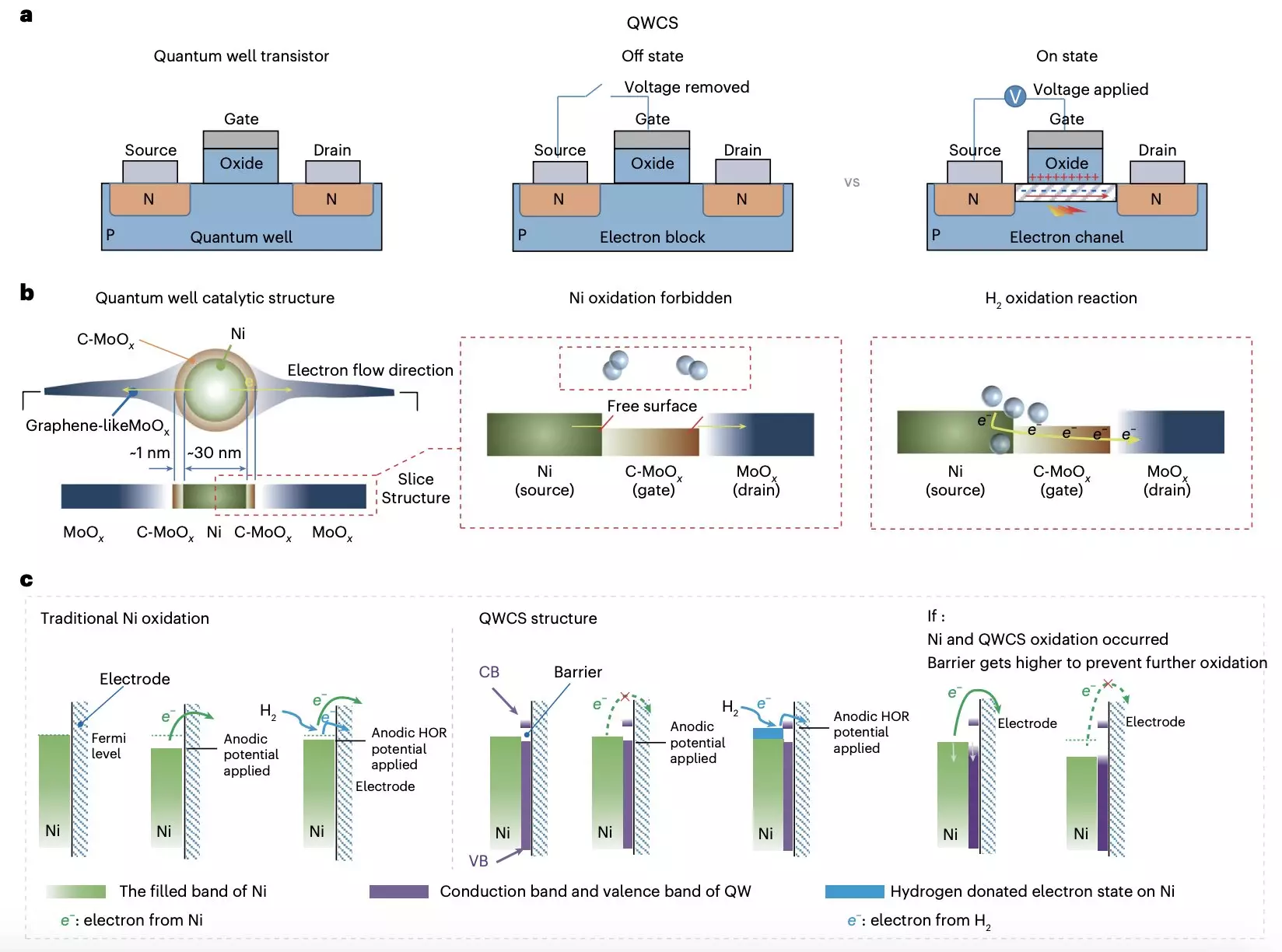
Researchers at Chongqing University and Loughborough University have developed a groundbreaking strategy to address the oxidation of metallic nickel electrocatalysts in AEMFCs. Their approach, detailed in a recent paper in Nature Energy, involves the use of a novel catalytic structure known as a quantum well-like catalytic structure (QWCS), which contains quantum-confined metallic nickel nanoparticles.
QWCSs are nanostructures that exhibit quantum well properties, enhancing catalytic activity. The researchers’ newly designed QWCS, Ni@C-MoOx, features nickel nanoparticles confined within a heterojunction of carbon-doped MoOx (C-MoOx) and amorphous MoOx. This structure enables the selective transfer of external electrons produced during the hydrogen oxidation reaction, preventing the electro-oxidation of the catalyst.
The Ni@C-MoOx catalyst has demonstrated impressive catalytic stability, maintaining excellent performance over 100 hours of continuous operation under harsh conditions. When integrated into an anode-catalyzed alkaline fuel cell, the catalyst achieved a specific power density of 486 mW mgNI-1, with no decline in performance after repeated shutdown-start cycles. This remarkable stability is attributed to the selective transfer of electrons facilitated by the QWCS design.
The innovative catalytic structure developed by the research team marks a significant step towards the development of cost-effective and reliable AEMFCs. By leveraging quantum confinement to prevent the electro-oxidation of non-precious metals, this design strategy has the potential to revolutionize fuel cell technology. Furthermore, the success of the Ni@C-MoOx catalyst opens the door to creating new catalysts with similar protective mechanisms, paving the way for enhanced performance and longevity in fuel cell applications.
The advancements in AEMFC technology represent a significant milestone in the quest for sustainable energy solutions. The use of quantum well-like catalytic structures to protect against catalyst degradation shows great promise for the future of fuel cells. By continuing to innovate and refine these designs, researchers can unlock the full potential of AEMFCs and accelerate the transition to a cleaner, more energy-efficient future.

Leave a Reply