Recent advancements in understanding the intricacies of biological systems have drawn interesting parallels with concepts traditionally situated within the realm of physics. A notable investigation conducted by researchers at São Paulo State University (UNESP) in Brazil has proposed a novel framework for analyzing protein compartmentalization in cells. Drawing from classical mixture theory, the study postulates that cells operate under a phase akin to the Griffiths phase known from magnetic systems. The integration of ideas from condensed matter physics into cellular biology highlights the growing trend of interdisciplinary approaches that foster new insights into the complex behaviors of living organisms.
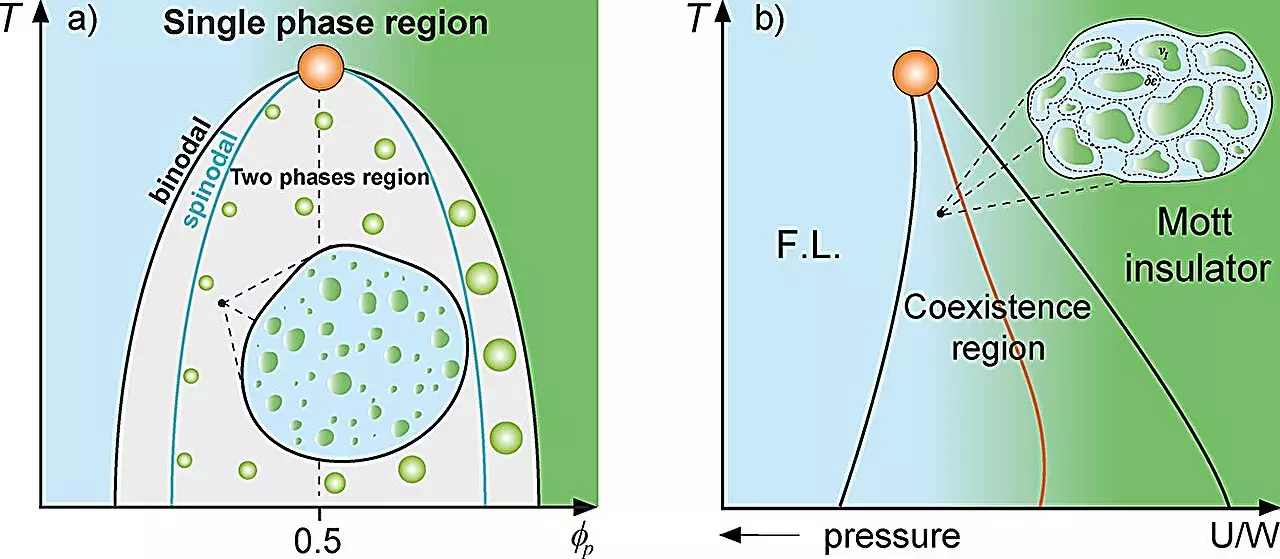
At the core of this investigation is the phenomenon of liquid-liquid phase separation, wherein proteins within cells condense into droplets under certain conditions. Such droplet formation resembles the “rare regions” observed in the magnetic Griffiths phase, wherein distinct magnetized and non-magnetized areas impact the overall dynamics of a system. The first author of the study, Lucas Squillante, along with Mariano de Souza, leads a compelling discussion that uncovers how variations in protein concentrations can give rise to significant changes in cellular behavior.
The researchers employed several thermodynamic models, including the Grüneisen parameter and the Flory-Huggins model, to elucidate the relationship between protein concentration and dynamics. They found a pronounced decrease in dynamics as the system approaches critical points such as the binodal line of phase separation. This insight reinforces the relevance of specific environmental conditions in determining the functional state of the cellular system.
What is particularly intriguing is the conceptual linkage established between the proposed Griffiths-like cellular phase and the origins of life. The researchers align their findings with historical theories put forth by Russian biochemist Aleksandr Oparin, suggesting that the survival of primordial organisms hinged on the slow dynamics of coacervates—droplets that formed from organic molecules. This historical context enriches the contemporary biological narrative, positing that understanding phase separation may elucidate fundamental evolutionary processes.
Furthermore, the aspect of homochirality—the concept that biological systems predominantly lean towards one type of chirality in molecules—has implications for the understanding of life’s genesis. By discussing chirality within the framework of their findings, the researchers open a dialogue about the molecular building blocks and their implications on the development of complex biological phenomena.
The relevance of this work extends far beyond the realm of theoretical physics and touches on pressing biomedical issues. A significant portion of the study discusses how liquid-liquid phase separation plays a critical role in various diseases, ranging from cancer to neurodegenerative disorders. The compartmentalization of proteins, particularly those tied to conditions like tumorigenesis, can significantly influence cellular functions, potentially leading to mutations and other pathologies.
The authors advocate for viewing protein droplet formation as a double-edged sword; while it may provide essential organizational mechanisms within cells, it can also contribute to pathological states under certain conditions. This bifurcation in understanding is crucial for devising therapeutic strategies that leverage the dynamics of cellular compartmentalization for clinical benefits.
As emphasized in the study, collaborative efforts between physicists and biologists are imperative for unraveling the complexities of life at the cellular level. The diverse expertise among the research collaborators—from geosciences to clinical medicine—demonstrates how different scientific disciplines can converge to foster innovation. By viewing biological phenomena through the lens of physics, researchers can gain new insights that might otherwise remain obscured within traditional disciplinary silos.
The implications of this research and its interdisciplinary nature are a testament to the evolving landscape of scientific inquiry. As we broaden our understanding of life through novel frameworks, the potential for transformative discoveries increases, promising a deeper comprehension of both the fundamental processes that govern living systems and the challenges posed by diseases.
The exploration of Griffiths-like cellular phases marks a significant step in bridging the gap between physics and biology. Through meticulous analyses and innovative models, researchers are redefining how we understand protein dynamics and their implications for life itself. As the dialogue between these two fields continues, it’s clear that the synthesis of ideas from various scientific domains can lead to profound advancements toward solving some of humanity’s most pressing biological challenges.

Leave a Reply