As energy demands escalate globally, researchers are in a relentless pursuit to innovate battery technologies. Their aim? To create batteries that not only hold more energy but also recharge with remarkable speed while maintaining longevity. An essential element contributing to a battery’s overall performance is its cathode material. In this domain, layered lithium-rich transition metal oxides have garnered significant attention. This article delves into these promising materials and explores the hurdles they face, offering insights from recent research studies.
Layered lithium-rich transition metal oxides present a compelling case for enhancing battery performance. Their unique layered structure facilitates the efficient movement of lithium ions during charging and discharging cycles, thereby amplifying energy storage capabilities. These materials are primarily composed of lithium, manganese, cobalt, and nickel, along with oxygen. The intricate interactions within this composition play a pivotal role in the redox reactions fundamental to battery operations. Simply put, these reactions enable batteries to either store energy when charged or release it during usage, underpinning their utility in everything from electric vehicles to portable electronics.
Moreover, the potential for higher energy density offered by these cathodes is a game-changer in the race to develop batteries that can power electric vehicles, making them more efficient and functional over longer distances. However, the benefits are coupled with critical challenges that have stunted their widespread adoption.
Despite their advantages, one of the most significant setbacks for layered lithium-rich transition metal oxides is their rapid degradation. Research indicates that these batteries tend to lose voltage over time, undermining their applications in key technologies. The marked instability shown by these cathodes during recharge cycles can lead to what researchers describe as a “vicious cycle” of performance plummeting. The findings from recent collaboration between institutions, including Sichuan University and the Southern University of Science and Technology, shed light on the degradation pathways that these materials undergo over time.
Published in *Nature Nanotechnology*, the study highlights that structural, chemical, kinetic, and thermodynamic factors contribute to the apparent short lifespan of batteries containing these cathodes. Researchers aimed to meticulously piece together how these materials transform on both nanoscale and microscale levels, hoping to reveal the hidden complexities that lead to failure.
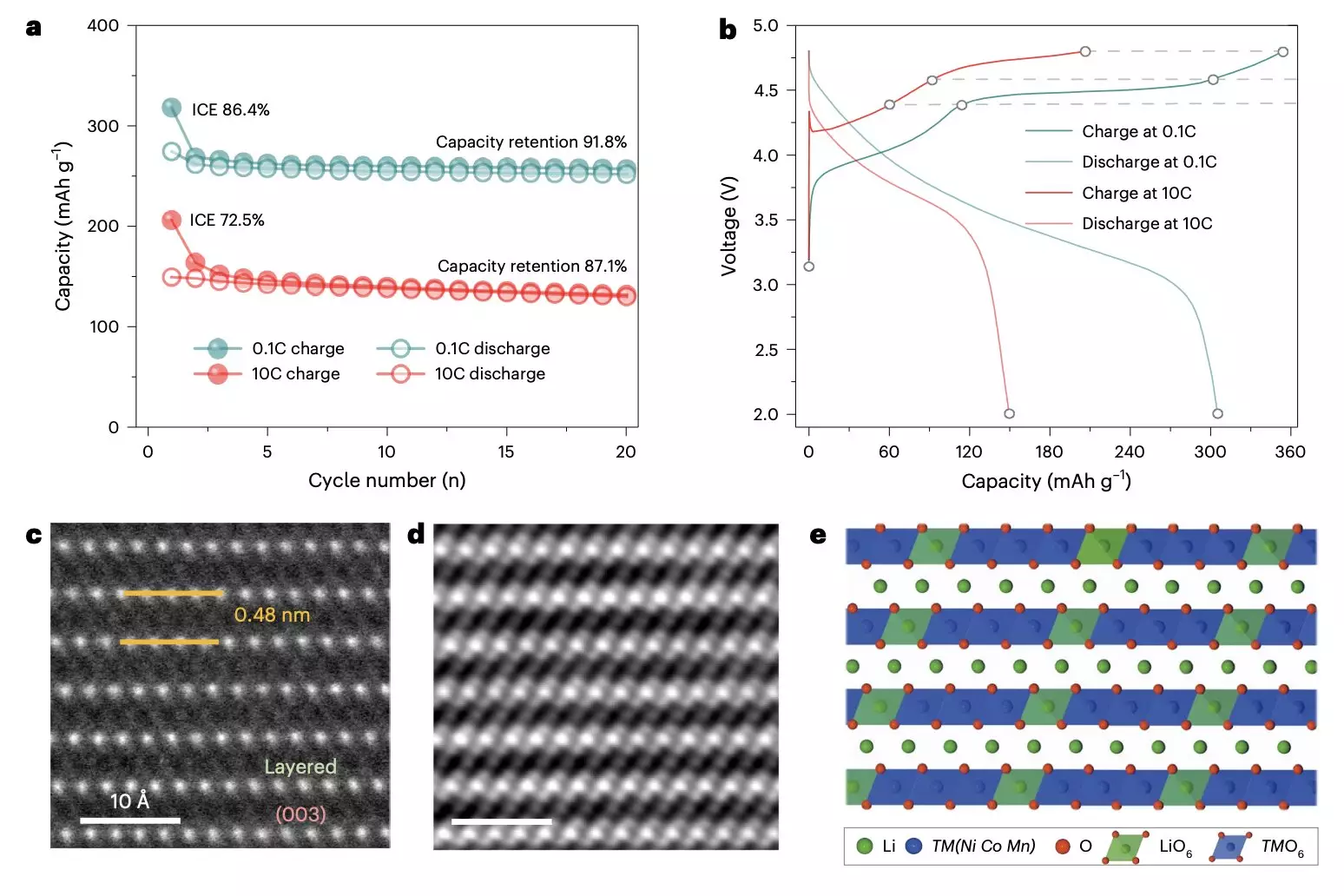
A Close Examination of Structural Changes
In their in-depth analysis, scientists employed advanced imaging techniques such as energy-resolved transmission X-ray microscopy (TXM). This sophisticated tool allowed for unparalleled visualization of structural and chemical compositions at the nanoscale. What emerged from the research were insights into oxygen defects and morphologies that significantly impact battery performance. For instance, the identification of varying degrees of oxygen defects highlighted a potential trigger for ongoing degradation.
The research team postulated that slow electrochemical activation results in extensive oxygen defects, which contribute to structural anomalies within the cathodes over repeated cycles of charging and discharging. These structural changes lead to a host of problems, including the formation of nanovoids, dissolution of transition metal ions, and disruption of lithium ion sites. Ultimately, these complications culminate in decreased efficiency and performance, which the industry strives to overcome.
The revelations from these recent studies illuminate the complex interplay of factors underpinning the performance of lithium-rich cathodes. By understanding the conditions that lead to their degradation, researchers are now tasked with developing targeted strategies to rectify these issues. Innovations in material design, the incorporation of stabilizing additives, or the redesign of existing cathode architectures could prove critical in overcoming these challenges.
As research continues to evolve and our understanding deepens, the potential for layered lithium-rich transition metal oxides to revolutionize battery technology remains within reach. Answering the call for better energy solutions is not merely a technological challenge but a quest that promises to reshape how we think about power consumption in our daily lives. With concerted efforts and advanced methodologies, the dream of efficient and enduring batteries could soon become a reality.

Leave a Reply