Recent advancements in neuroscience are illuminating the potential of non-invasive techniques to treat debilitating neurological disorders. Among these innovations is transcranial focused ultrasound (tFUS), which employs high-frequency sound waves to stimulate targeted areas of the brain. This state-of-the-art method shows particular promise for individuals suffering from drug-resistant epilepsy and various neurological conditions that manifest recurrent tremors. A group of researchers from notable institutions including Sungkyunkwan University, the Institute for Basic Science, and the Korea Institute of Science and Technology is making significant strides in refining this approach by developing a sophisticated sensor designed to enhance the efficacy of tFUS in clinical applications.
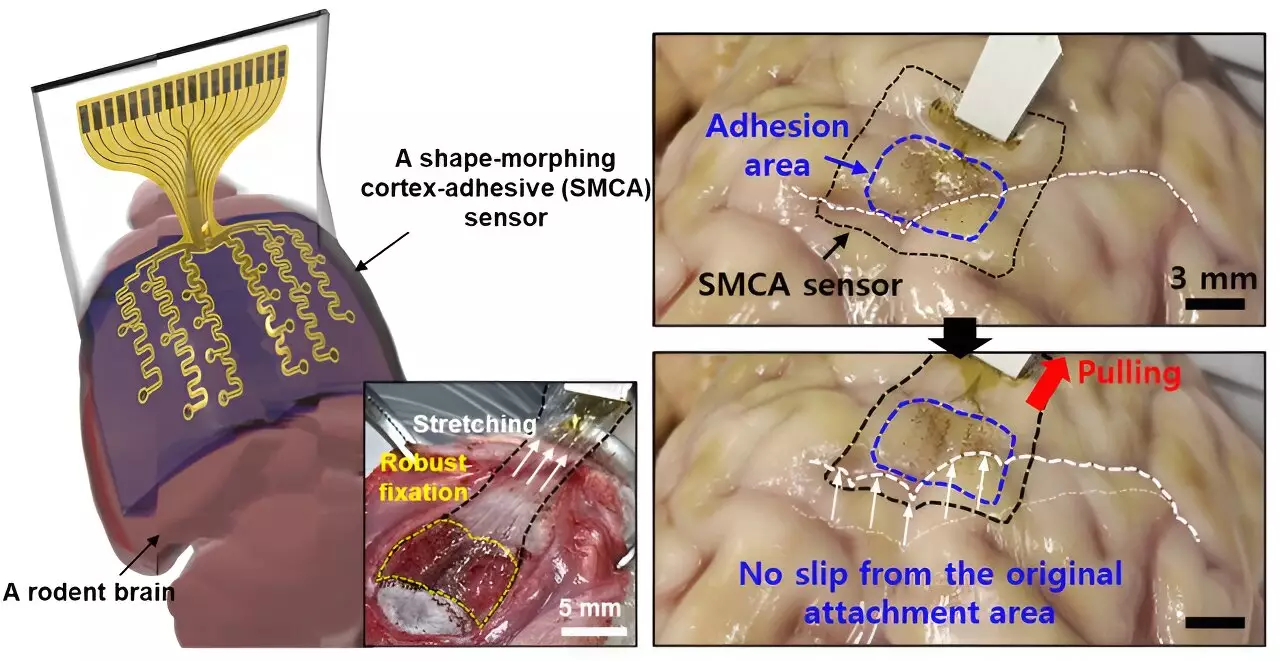
Traditionally, sensors that interface with the brain surface have encountered significant challenges, particularly their inability to achieve a snug fit over the brain’s intricate and irregular surface. The consistency and precision of neural signal measurements have previously been compromised due to this incompatibility. Donghee Son, the lead author of the research, articulated these limitations, explaining how prior sensors struggled to provide accurate readings as they detached or slipped from their original positions due to micro-movements of the brain and fluctuations in cerebral spinal fluid. Such shortcomings have critical implications for diagnosing conditions and understanding brain lesions effectively.
The prior work by distinguished researchers, John A. Rogers and Dae-Hyeong Kim, while commendable, showcased these inherent issues. Even with a design that prioritized thinness and flexibility, the reality of brain curvature often led to insufficient adhesion, limiting long-term monitoring. This new research confronts these obstacles head-on by creating a sensor capable of maintaining a secure position on the complex surface of the brain, ultimately improving the reliability and duration of data collection.
The innovation presented by Son and his dedicated research team is nicknamed the ECoG sensor. This sensor sets new benchmarks in brain interaction by allowing for tight conformability to the contours of the brain tissue. As the team optimizes its adhesive capabilities, the ECoG sensor promises a meticulous collection of neural signals while diminishing the interference from mechanical noise and other extraneous vibrations. This feature is particularly crucial for enhancing treatment outcomes for patients undergoing low-intensity focused ultrasound, as it mitigates the challenges posed by variations in individual neurological conditions.
The design is formidable. Composed of three primary layers, the ECoG sensor integrates a hydrogel for robust tissue bonding, a self-healing polymer that adapts its shape as needed, and a highly flexible layer featuring gold electrodes and interconnects. Upon application, the hydrogel component swiftly undergoes a gelation process, fostering a strong bond with the brain tissue. This not only promotes immediate stable attachment but also enhances operational efficacy across extended periods.
As the field of neurology pivots toward personalized medicine, the ECoG sensor emerges as a pivotal tool. The research underscores an essential focus on creating individual-centric treatment plans for conditions like epilepsy, where real-time monitoring of brain activity is fundamental to tailoring interventions. The noise reduction capabilities of the ECoG sensor significantly alleviate the impediments previously encountered by conventional surface-attached sensors, enabling consistent and real-time analysis during focused ultrasound interventions.
Son’s team envisions that this breakthrough technology will bolster the therapeutic landscape not just for epilepsy, but also for a wider spectrum of neurological disorders. The adaptability of the ECoG sensor to diverse brain contours marks a transformative development, reinforcing the importance of continuous data collection in informing therapeutic adjustments based on patient-specific responses.
Excitingly, initial tests of the ECoG sensor have been conducted on awake rodents, yielding highly promising results in terms of seizure control and brain wave measurement. These discoveries lay the groundwork for further development. The research team aims to enhance the sensor’s capabilities by expanding the electrode array, thereby allowing for high-resolution mapping of brain signals crucial for understanding complex neurological conditions.
Upon successfully navigating clinical trials, the ECoG sensor could change the landscape of neurological treatment modalities, not only facilitating refined therapeutic approaches for epilepsy but also supporting the evolution of advanced prosthetic technologies. The collaborative efforts spearheaded by Son and his colleagues emphasize a future where personalized and effective treatment strategies are not just a goal but an achievable reality.
With the ECoG sensor, we stand on the cusp of a new era in neurological treatment—one where innovation meets clinical necessity, ensuring that patients receive targeted care tailored to their precise needs. This research is set to unleash mechanisms of healing and understanding that may redefine our approach to brain disorders and their management.

Leave a Reply