In the delicate balance of nature, photosynthesis serves as an essential process, whereby plants and certain bacteria convert sunlight into chemical energy. This remarkable transformation mirrors the function of solar photovoltaics, which convert light into electricity through the movement of electrons. At the core of both processes lies charge transfer—an interaction governed at the molecular level by swift electronic motion. Understanding the intricacies of electron and charge transfer processes can unlock doors to optimizing energy conversion technologies and provide a foundation for future research in molecular science.
When a molecule absorbs light, an instantaneous but complex phenomenon occurs: the redistribution of electronic density within that molecule. This ultrafast event engages various quantum effects and chains of molecular dynamics that occur on incredibly short timescales, sometimes as brief as femtoseconds or even attoseconds. These scales are so diminutive that they approach the fundamental limits of time perception in physical science. Thus, enhancements in measurement techniques, particularly those able to capture quick temporal resolution, have become increasingly vital. Such measurements not only demystify the foundational principles behind these physical mechanisms but also provide pragmatic insights into how we can manipulate the chemical and structural traits of molecules for better performance in light-mediated reactions.
Recent advancements have heralded the use of ultrashort ultraviolet pulses, particularly those generated by high-order harmonic sources or free electron lasers. These innovative tools allow researchers to initiate and scrutinize molecular responses to photoionization with extraordinary precision. A noteworthy study conducted by experts at Politecnico di Milano and various prestigious institutions across Madrid exemplifies the remarkable capability of these techniques. The research, published in *Nature Chemistry*, emphasizes the cutting-edge deployment of attosecond extreme-ultraviolet pulses to explore the subtle dynamics of electron transfer mechanisms within molecular systems.
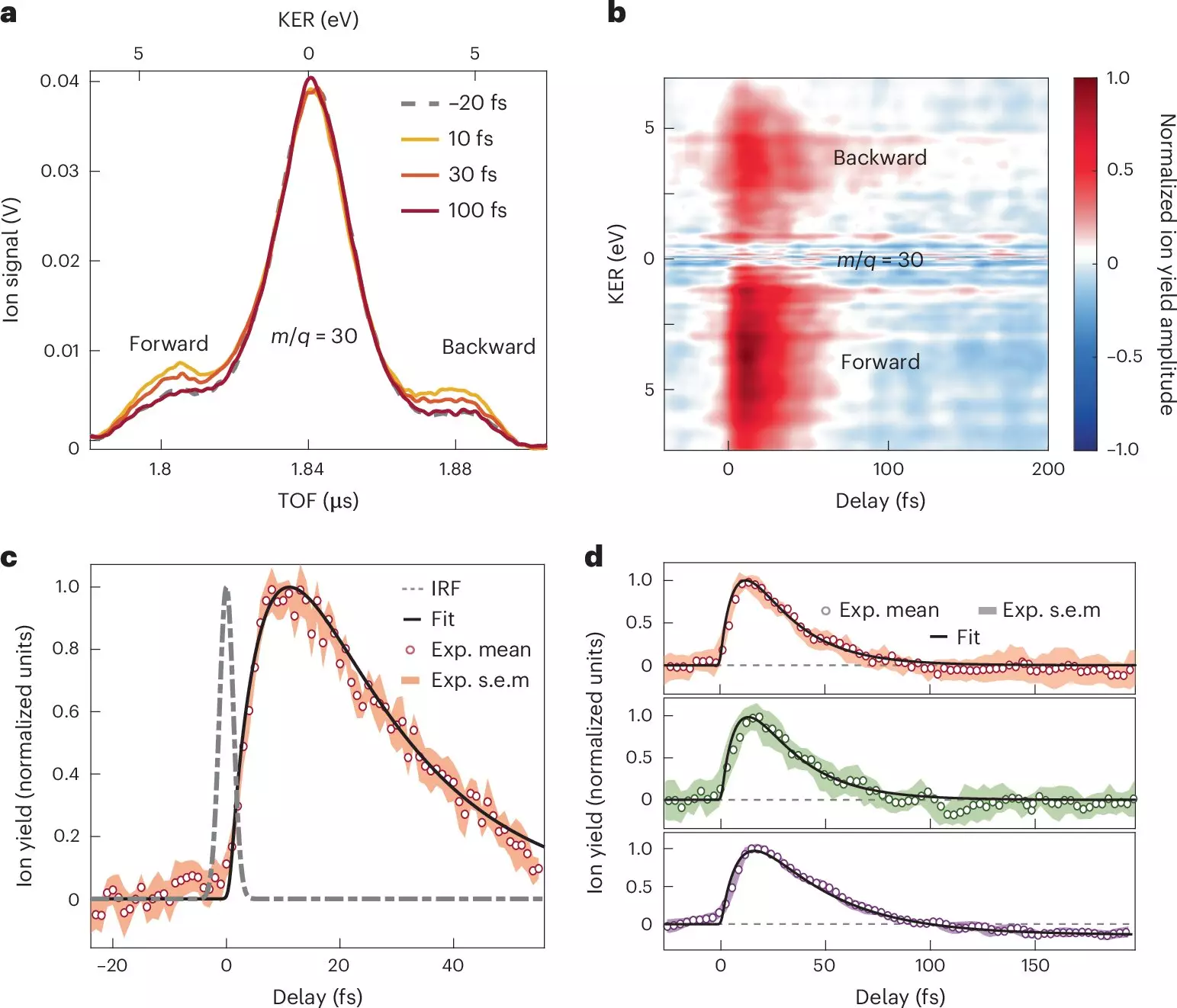
In their investigation, the research team focused on nitroaniline molecules, exposing them to attosecond pulses. The results were pathbreaking, leading to unprecedented insights into the early stages of charge transfer. Utilizing advanced methodologies, including attosecond extreme-ultraviolet pump and few-femtoseconds infrared probe spectroscopy, alongside sophisticated many-body quantum chemistry calculations, the researchers uncovered intricate details of the rapid sequential steps involved in electron dynamics.
One of the standout conclusions from the study indicates that the electron transfer from the amino donor group occurs in less than ten femtoseconds. This remarkably swift movement is influenced by a coherent coupling between the electrons and the nuclei, a revelation that holds profound implications for how we envision electron donor-acceptor systems.
The journey of charge transfer does not merely stop at the transfer itself; it is followed by a relaxation phase that transpires over approximately thirty femtoseconds. During this era, the nuclear wave packet expands within the excited electronic states of the molecular cation, showcasing how interconnected nuclear and electronic motions shape charge migration processes. These findings illuminate the synchronized relationship between electronic motion and structural changes, offering a redefined understanding of what occurs during photoionization events.
The insights gained from this groundbreaking research hold great promise for both theoretical frameworks and practical applications in the field of attosecond science. By elucidating the timings and spatial dynamics associated with charge migration in organic molecules, the researchers have constructed a theoretical foundation that can refine existing models and educational frameworks surrounding charge transfer phenomena.
As we advance into an era of nanotechnology and sophisticated molecular engineering, the potential applications of this knowledge are vast. Understanding ultrafast electron dynamics can empower the development of more efficient photovoltaic materials, semiconductors, and beyond.
The study of ultrafast electron dynamics represents a pivotal shift in our comprehension of molecular behavior under light exposure. The capacity to observe and manipulate these processes with attosecond precision could very well revolutionize the field of chemistry and materials science. As researchers continue to unveil the complexities governing electron and charge transfer, we stand at the threshold of new frontiers in energy conversion technologies and molecular manipulation strategies—promising a future where our grasp of the quantum world can lead to significant advancements in both theory and application.

Leave a Reply