As the world grapples with the adverse effects of climate change, innovative solutions for reducing carbon dioxide (CO2) emissions are becoming increasingly critical. One promising avenue lies in the advanced techniques of artificial photosynthesis, which aim to mimic the natural processes of plants to convert CO2 into valuable fuels. A groundbreaking system developed at the University of Michigan demonstrates exceptional capabilities in this field by chaining carbon atoms—specifically, it successfully synthesizes ethylene, a key hydrocarbon used in various industries, including plastics.
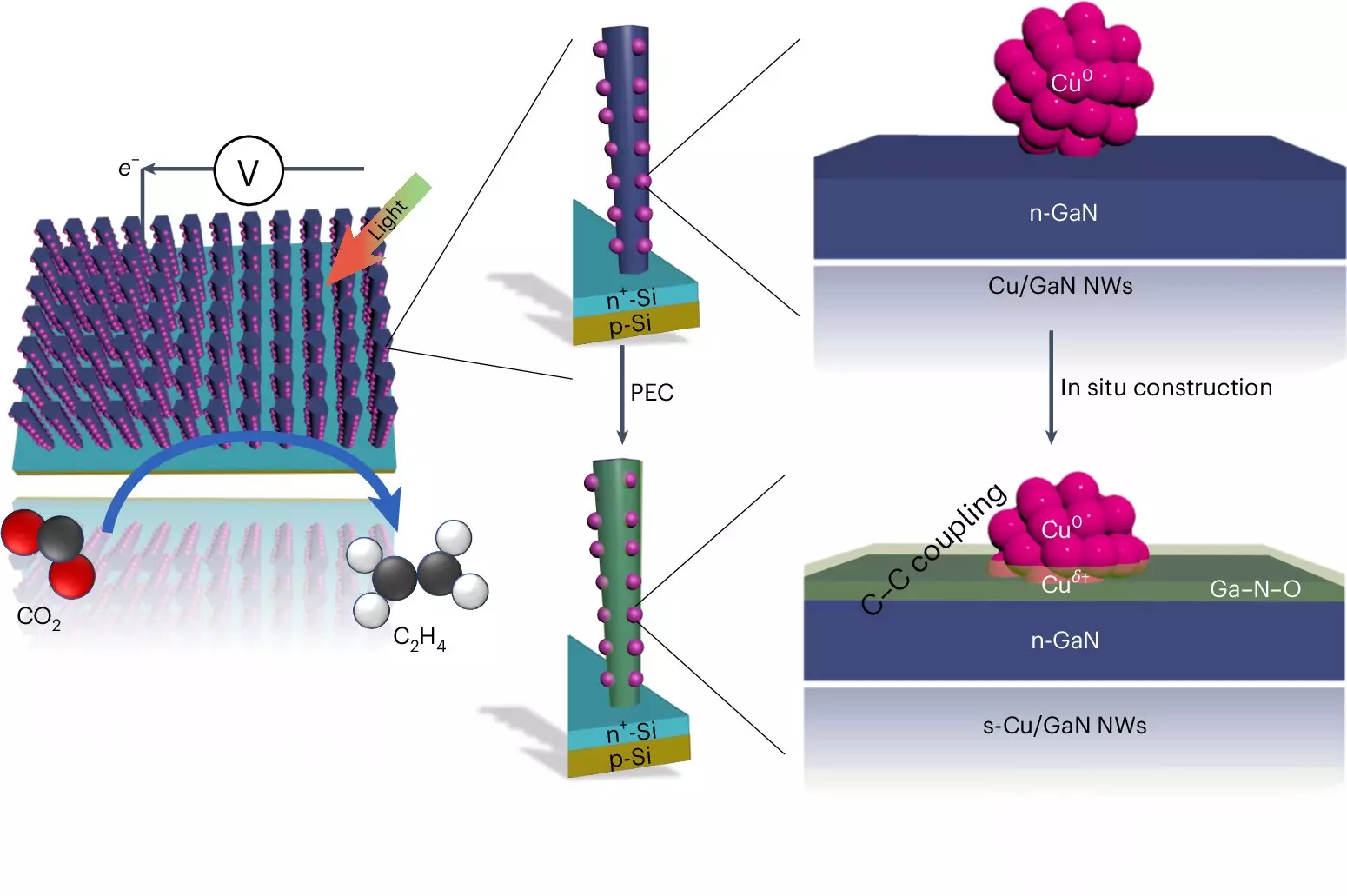
The artificial photosynthesis system designed by the University of Michigan relies on an intricate interplay of advanced materials and chemical reactions. Central to this technique is the ability to combine carbon atoms from CO2 with hydrogen atoms derived from water (H2O), a process that typically poses challenges, especially in ensuring that oxygen is effectively removed from the reaction. The cutting-edge device employs two types of semiconductors: gallium nitride nanowires, which are astonishingly thin, and a silicon substrate. This configuration allows the system to effectively absorb sunlight, harnessing solar energy to initiate the complex chemical reactions necessary for converting CO2 and water into ethylene.
During the process, light photons excite electrons within the gallium nitride nanowires, facilitating the conversion of water molecules. This reaction generates molecular hydrogen and oxygen, with the gallium nitride being able to absorb excess oxygen and transition into a gallium nitride oxide state. The copper clusters lining the nanowires play a pivotal role in this process as well—these clusters capture hydrogen and facilitate its bonding with carbon from CO2, ultimately forming ethylene.
The performance of the University of Michigan’s artificial photosynthesis system surpasses existing technologies, boasting five to six times the efficiency of conventional methods that rely on solar energy for carbon dioxide reduction. The system not only demonstrates remarkable yields of ethylene but also maintains longevity; it has functioned continuously for an impressive 116 hours without notable drops in performance, and similar units have lasted up to 3,000 hours.
Comparatively, other catalytic systems—such as those based on silver and copper—while efficient, often require complex fluids for operation and exhibit significantly short operational lifespans, deteriorating after just a few hours. This stark contrast underscores the robustness of the Michigan team’s innovation, which benefits from the synergy achieved between gallium nitride and the water-splitting reactions. The addition of oxygen produced during the process appears to enhance the catalyst’s efficacy and supports a self-healing mechanism that improves durability.
Ethylene, while a fundamental building block in the production of countless plastic products, is conventionally derived from fossil fuels through high-temperature and high-pressure processes that inevitably emit CO2. By capturing and recycling atmospheric CO2 into useful compounds, the Michigan system holds tremendous potential for mitigating greenhouse gas emissions associated with plastic production.
Moreover, the ultimate goal extends beyond ethylene production. As researchers refine the system, they aspire to produce longer-chain hydrocarbons, which can serve as liquid fuels, thereby contributing to the transition to greener transportation solutions. For instance, creating propanol—a three-carbon compound—could pave the way for sustainable alternatives in various forms of transport that currently depend on gasoline or diesel.
Despite the promising results, the pursuit of optimizing this system is ongoing. Researchers are keen on further dissecting the limits of the device’s longevity and overall efficiency. The goal is to harness even greater yields of longer carbon chains while ensuring the sustainability of the process under varied operational conditions. The challenge of effectively removing oxygen continues to be crucial, as any inefficiencies in this stage could hinder the overall process.
The breakthrough achieved by the University of Michigan’s artificial photosynthesis system represents a significant stride toward sustainable fuel production. By converting CO2 into ethylene with unprecedented efficiency and durability, this technology not only addresses the pressing need to reduce greenhouse gas emissions but also opens new avenues for creating environmentally friendly alternatives to fossil fuels. As advancements continue, the potential to transform how we produce energy and materials while preserving the planet becomes ever more tangible, urging collective efforts towards a sustainable future.

Leave a Reply